
No response returned

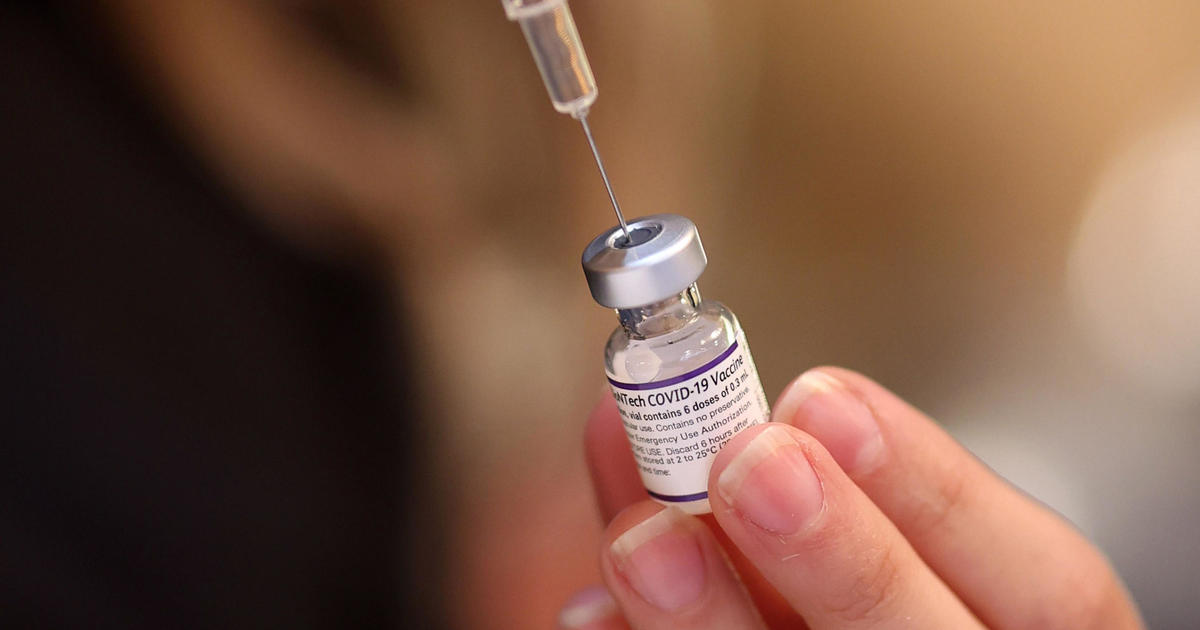
The Food and Drug Administration Wednesday authorized updated COVID-19 vaccines, but limited their use for most age groups and rescinded the that made shots available for healthy young children.
Vaccines from Pfizer, Moderna and Novavax are approved for all seniors, but only for younger adults and children with health conditions, creating barriers for healthy adults and children to get vaccinated.
The decision means there is no longer a COVID vaccine authorized for healthy children under 5 years old. Previously, Pfizer's shot was authorized for that use. The Moderna vaccine has only been approved for children with health conditions, and the Novavax vaccine is not available for children under 12 years old.
In a statement to last week, a Pfizer spokesperson said the decision was "not related to the safety and efficacy of the vaccine which continues to demonstrate a favorable profile."
The American Academy of Pediatrics called the FDA's decision "deeply troubling."
"As we enter respiratory virus season, any barrier to COVID-19 vaccination creates a dangerous vulnerability for children and their families. Respiratory illnesses can be especially risky for infants and toddlers, whose airways and lungs are small and still developing," Susan Kressly, president of the AAP, said in a statement.
Since insurers generally cover only federally recommended vaccines, without the EAU for healthy children, the vaccination would likely be an out-of-pocket copay for the full cost of the vaccine — around $200 — for families who still want their children to get the shot, said Jayne Hornung, chief clinical officer of health care-focused market partner MMIT.
"Notably, certain health systems or insurers like Blue Shield of California and Kaiser Permanente have stated publicly to continue covering COVID-19 vaccines in all age groups, even when federal recommendations have shifted," Hornung said.
The emergency authorization decision comes after the American Academy of Pediatrics released that, for the first time in 30 years, differ from U.S. government advice.
In published Aug. 19, the AAP is "strongly recommending" COVID-19 shots for children ages 6 months to 2 years old. For older children, shots are also advised but up to parents' discretion, the AAP said.
The Centers for Disease Control and Prevention's advice is different. Under Health Secretary Robert F. Kennedy Jr., the CDC doesn't recommend COVID-19 shots for healthy children of any age, but instead, says in consultation with a physician.
Kennedy that the shots would no longer be recommended for healthy kids, a decision that health experts have said lacks scientific basis. The AAP COVID-19 shots for children older than 6 months.
Dr. Céline Gounder, TheNews medical contributor and editor-at-large for public health at KFF Health News, says there's "a lot of noise out there" when it comes to vaccines.
"Parents should really stick the course and make sure that their children get all of the routine childhood vaccinations," she
The LP.8.1 strain, which is the target of the updated vaccines for the upcoming season, has been abundant in the U.S. and other regions since earlier this year.
As summer travel picked up, however, showed the , nicknamed "Stratus," is predominant in the U.S.
While symptoms of the variants are largely the same a previous COVID infections, Stratus has gained some attention for what The Associated Press reported as . Dr. Eric Asher earlier this month that patients with the variant are coming in with a hoarse voice.
Still, as with , experts say cases aren't any more severe — they can just be highly contagious as the virus mutates.





